Ion/Substrate Transport and GPCR Signaling
The extension of the hybrid-solvent CpHMD for transmembrane proteins has opened a door for obtaining previously unattainable insights into biological proton/ion transport. To pave the way, we demonstrated that CpHMD can directly reveal the atomic details of the pH-dependent conformational transitions of a proton channel, an antiporter, and a multi-drug efflux pump. Most recently, we have begun to explore the molecular mechanisms of ligand-receptor recognition and activation.
Mahinthichaichan P, Liu R, Vo QN, Ellis CR, Stavitskaya L*, and Shen J*
2023Structure-Kinetics Relationships of Opioids from Molecular Dynamics Simulations and Machine Learning
J Chem Inf Model 63: 2196–2206, 2023
PMCID: PMC10028827 DOI: 10.1021/acs.jcim.3c00069
Mahinthichaichan P, Vo Q, Ellis CR, and Shen J*
2021Kinetics and Mechanism of Fentanyl Dissociation from the mu-Opioid Receptor.
JACS Au 1: 2208–2215, 2021
PMCID: PMC8715493 DOI: 10.1021/jacsau.1c00341
Vo Q, Mahinthichaichan P, Shen J*, and Christopher R. Ellis*
2021How μ-opioid receptor recognizes fentanyl.
Nat Commun 12: 984, 2021
PMCID: PMC7881245 DOI: 10.1038/s41467-021-21262-9
Huang YD, Henderson JA and Shen J*
2021Continuous constant pH molecular dynamics of transmembrane proteins.
Methods Mol Biol. in press, 2021
PMCID: None DOI: 10.1101/2020.08.06.239772
Henderson JA, Beckstein O, and Shen J*
2020Alternative proton-binding site and long-distance coupling in Escherichia coli sodium–proton antiporter NhaA.
Proc Natl Acad Sci USA, 117: 25517-25522, 2020
Shen J*
2018Zooming in on a small multidrug transporter reveals details of asymmetric protonation.
Proc Natl Acad Sci USA 115: 8060-8062, 2018
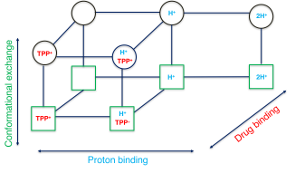
Nature is efficient; why would it require two protons if one is enough to do the job? This commentary article discusses a recent computational work in the context of our current understanding of how proton translocation mediates the active transport of drugs and substrates across the biological membrane.
Yue Z, Chen W, Zgurskaya, H, and Shen J*
2017Constant pH molecular dynamics reveals how proton release drives the conformational transition of a transmembrane efflux pump.
J Chem Theory Comput 13: 6405-6414, 2017
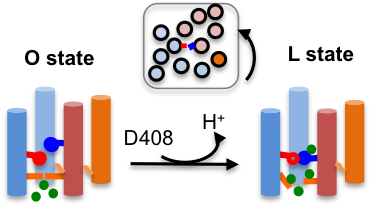
AcrB is the inner-membrane transporter of the AcrAB-TolC efflux complex in E. coli which confers resistance to antibiotics. Crystal structures have revealed three distinct states in the drug transport cycle; however, detailed mechanism remains unknown. Our study offered convincing support for the 1:1 drug:proton stoichiometry by depicting how proton release triggers the conformational transition, in agreement with the crystal structure.
Chen W, Huang YD, and Shen J
2016Conformational activation of a transmembrane proton channel from constant pH molecular dynamics.
J Phys Chem Lett 7: 3961-3966, 2016
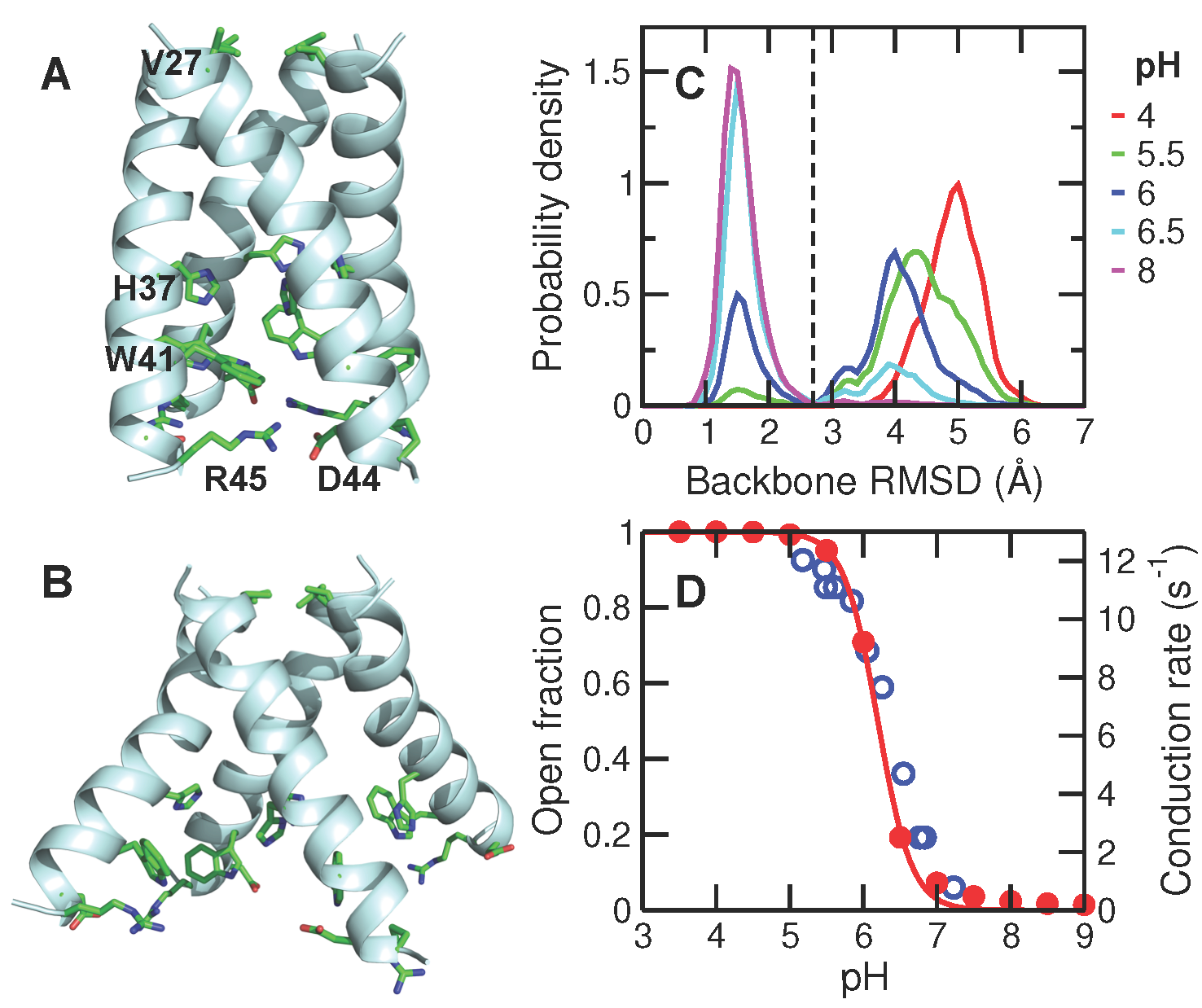
Proton-coupled transmembrane proteins play important roles in human health and diseases. We performed the first computer simulation that 1) directly describes the proton-coupled conformational activation of a transmembrane channel with fully atomic detail; 2) accurately determines the stepwise acid-base constants of a transmembrane channel; 3) provides the proton-coupled free energy of channel activation. The presented methodologies and major findings are generalizable for studies of proton-coupled channels and transporters.
Huang YD, Chen W, Dotson DL, Beckstein O, and Shen J*
2016Mechanism of pH-dependent activation of the sodium-proton antiporter NhaA.
Nat Commun 7: 12940, 2016
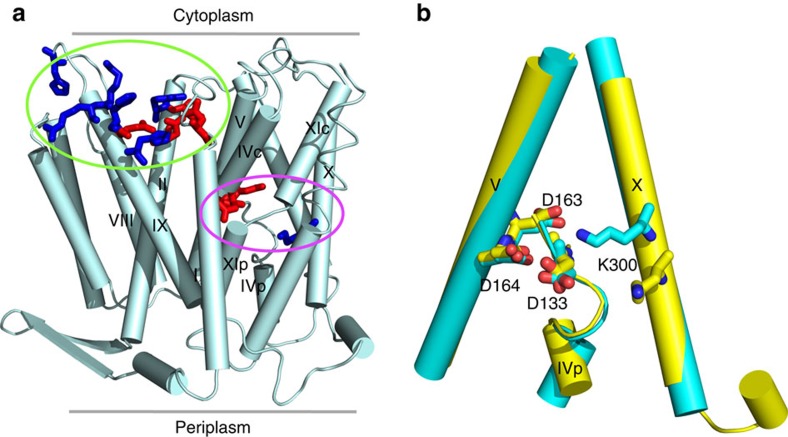
Escherichia coli NhaA is a prototype sodium-proton antiporter, which has been extensively characterized by X-ray crystallography, biochemical and biophysical experiments. However, the identities of proton carriers and details of pH-regulated mechanism remain controversial. Here we report constant pH molecular dynamics data, which reveal that NhaA activation involves a net charge switch of a pH sensor at the entrance of the cytoplasmic funnel and opening of a hydrophobic gate at the end of the funnel. The latter is triggered by charging of Asp164, the first proton carrier.